News
AKI awarded ISO 13485:2003 medical device quality standard
12 Jan 2007
12 Jan 2007
Injection moulding specialists A K Industries has been awarded the prestigious Quality Management Standard for Medical Devices ISO 13485:2003 at the first assessment. The Hereford based company has always seen the pursuit of the highest quality standards as key to success and this latest qualification confirms their commitment to the medical components market. The preparation and evaluation process took place at the same time as AKI was introducing a new clean room operation for a new customer, so the success was a double bonus.
Biotrace International, part of the 3M Group, recently turned to AKI for a two shot injection moulded clear polypropylene component. Biotrace is acknowledged as the leading global brand in rapid hygiene monitoring. Their systems offer rapid, simple and reliable solutions for verifying cleanliness or measuring microbial contamination in diverse areas such as food production/preparation, environmental and industrial processes or healthcare facilities.
To achieve the necessary component specification a cleanroom manufacturing facility was required. AKI have built a dedicated manufacturing cell for Biotrace which includes an Arburg 470C 1500-350 two shot injection moulding machine as the component also requires a moulded-in TPE seal. Joint Managing Director Keith Williams was delighted. “To have succeeded in achieving this quality standard while introducing new products and maintaining production levels put everyone under pressure but the whole team at AKI gave their 100% support and demonstrated, once again, why AKI continues to be successful”.
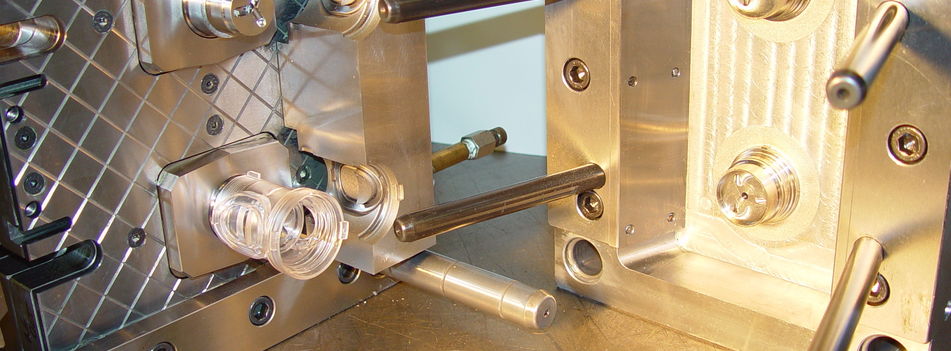
Many of the mould tools we run in our moulding machines have complex cores which operate in a plane 90 degrees to the tool opening plane.